Objective
Reduce the number of layers (windings) by using thick electrodes for lithium secondary battery
→ Increase the amount of active material due to a decrease of number of separators and current collectors
Improved energy density of the battery!
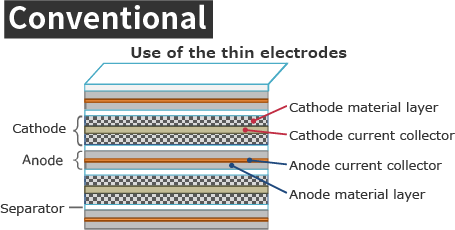
Conventional lithium secondary batteries have thin electrodes stacked (or wound).
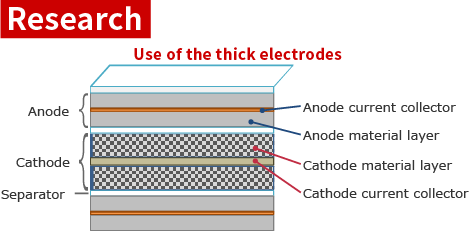
If thick electrodes are used, the number of layers (windings) can be reduced.
→ Since the ratio of active material layer can be increased by reducing the number of separators and current collectors, an improvement in the energy density of the battery can be expected.
Problems of the conventional (coating) thick electrode
- Deterioration of battery performances due to cracking, exfoliation, or dropping of the active material layer from current collector
- Degradation of battery performances due to increase in electrode resistance
Proposal of new thick electrode
[Conventional] Applying active material mixture to current collector foil
[New] Filling current collector with three-dimensional porous structure
SEM image of cross-section of cathode
Cathode using conventional Al foil current collector (cathode applied on one side)
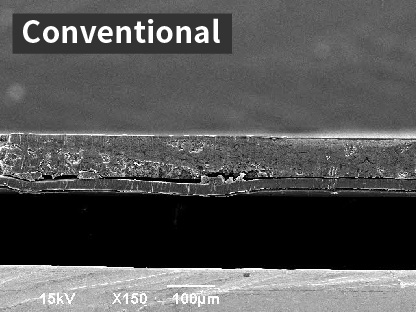
A cathode layer is formed on the Al foil. A part of the exfoliation is confirmed.
Cathode using three-dimensional porous Al current collector
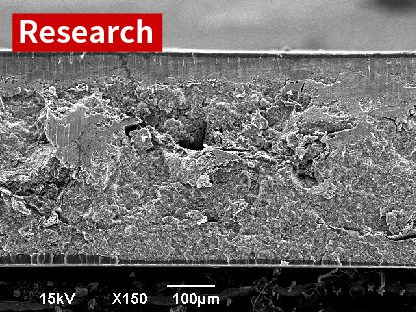
Al current collector and cathode material are fine mixed.
| Current collector | Thickness | Capacity |
|---|---|---|
| Al foil | 100μm | 2.0mAh/cm2 |
| Porous Al | 400μm | 8.4mAh/cm2 |
Electrode characteristics of LiFePO4 (LFP; Lithium iron phosphate) cathode material
LiFePO4
Charge / discharge curve of LFP cathode (half cell)
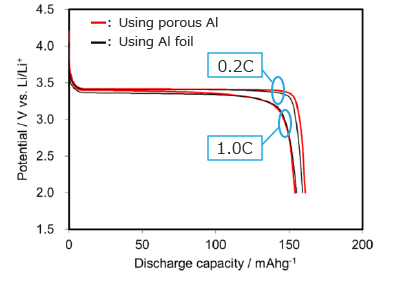
Cathode using porous Al current collector has the same discharge performance as using Al foil current collector.
LiFePO4
Cycle characteristics of LFP / graphite cell
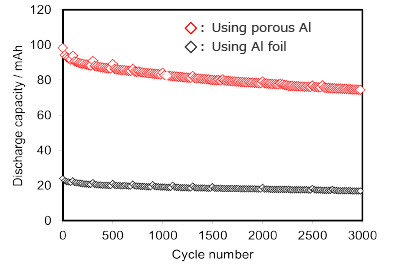
Cathode using porous Al current collector is equivalent to or better than it using Al foil current collector.
