Objective
- With the graphite anode conventionally used in lithium secondary batteries, it is difficult to further increase the energy density.
- The thick cathode, which is expected to have higher energy density, has a larger capacity per area, and the conventional graphite anode cannot be balanced as a counter electrode.
→ High capacity Li metal should be used as an anode material.
Improve battery energy density!
Technology for mastering Li metal anodes -1
The 3DOM separator (3DOM: Three Dimensionally Ordered Macroporous) is used to uniformly react lithium metal.
Characteristics
- High porosity
- Ordered pore structure
(Note)
The 3DOM separator was developed by a research group of Professor Kanamura at Tokyo Metropolitan University.
Currently, preparing for mass production at 3DOM Inc. (https://www.3dom.co.jp/en/)
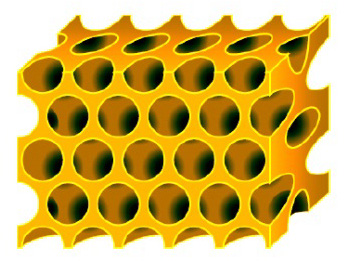
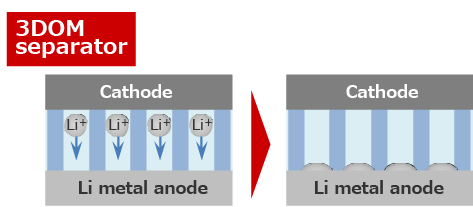
Since the pores exist uniformly, the Li metal anode also reacts uniformly.
Since Li plates uniformly, Li dendrite is difficult to grow.
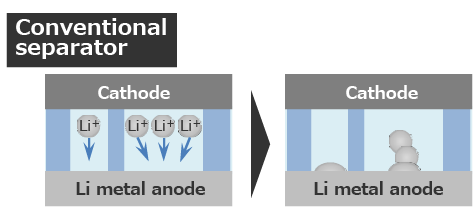
Since the pores are non-uniformly, the Li metal anode also reacts non-uniformly.
Since Li plating sites are concentrated, Li dendrite is easy to grow.
Technology for mastering Li metal anodes -2
Lithium secondary batteries generally start from charging.
However, since the surface of initial Li metal is covered with a passivation film, the reaction becomes non-uniform and Li plates locally.
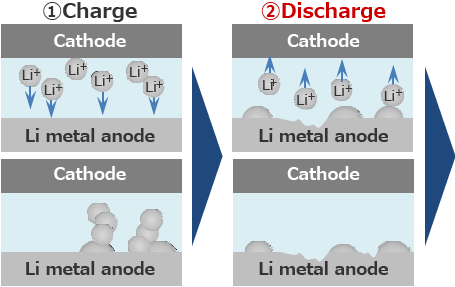
Since Li plates non-uniformly, it tends to be dendritic.
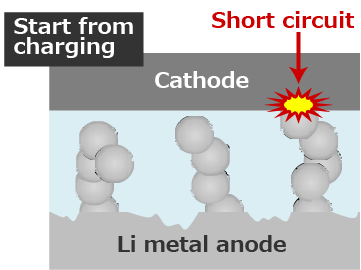
When the lithium metal anode is repeatedly charged and discharged, Li Plating/Stripping reaction occurs at the same location, and the growth of Li dendrite is promoted.
Lithium secondary batteries start from discharging.
Since the passive film is removed first, Li is easily plated uniformly.
However, it is necessary to improve the cathode to prepare with the discharge start!
(Note) The conventional cathode materials (LiCoO2, LiNiO2, LiFePO4, ...) cannot be discharged because they are in a discharged state. It is made possible by adding a dischargeable material such as MnO2.
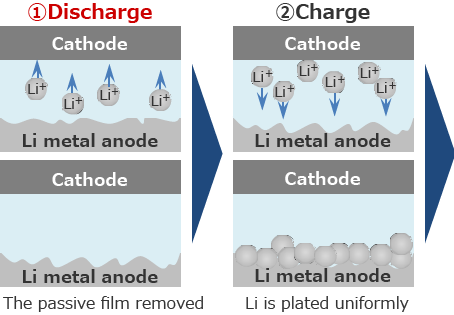
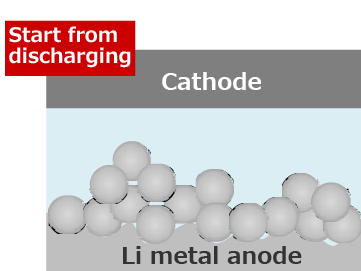
When the lithium metal anode is charged and discharged repeatedly, Li Plating/Stripping reaction occurs at the same location, and the growth of Li dendrite is suppressed.
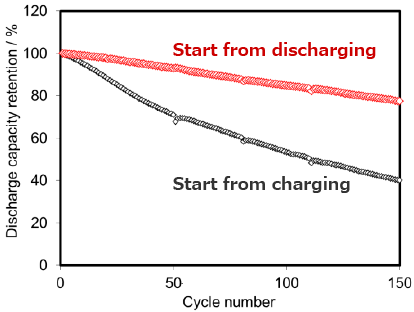
Problem of lithium metal anode
Short life due to Li dendrite
